Recent advances in the life sciences industry are undoubtedly improving patient outcomes — and increasing the cost, complexity, and duration of clinical trials. These trends have led to mounting pricing pressure from governments and payers, prompting R&D leaders to re-evaluate their operations and seek ways to drive efficiency without compromising quality.
One core capability consistently under scrutiny is resource management (RM). Leaders must navigate constrained budgets and rising expectations for trial speed, flexibility, and workforce optimization. In this environment, the ability to separate myth from fact when assessing workload, capacity, and capabilities is critical.
Effective resource management delivers measurable value. It gives organizations visibility into who is available, what expertise they bring, and where they can be most impactful. It empowers leaders to make informed, timely decisions about how to deploy their teams across functions. Most importantly, it ensures that the right people — with the right skills — are assigned to the right work at the right time.
To meet the demands of today’s trials, organizations need more than decentralized headcount tracking. They need a centralized, analytics-enabled resource management capability — one that provides full transparency into bandwidth, talent allocation, and strategic priorities across functions.
Current trends: resource management implications
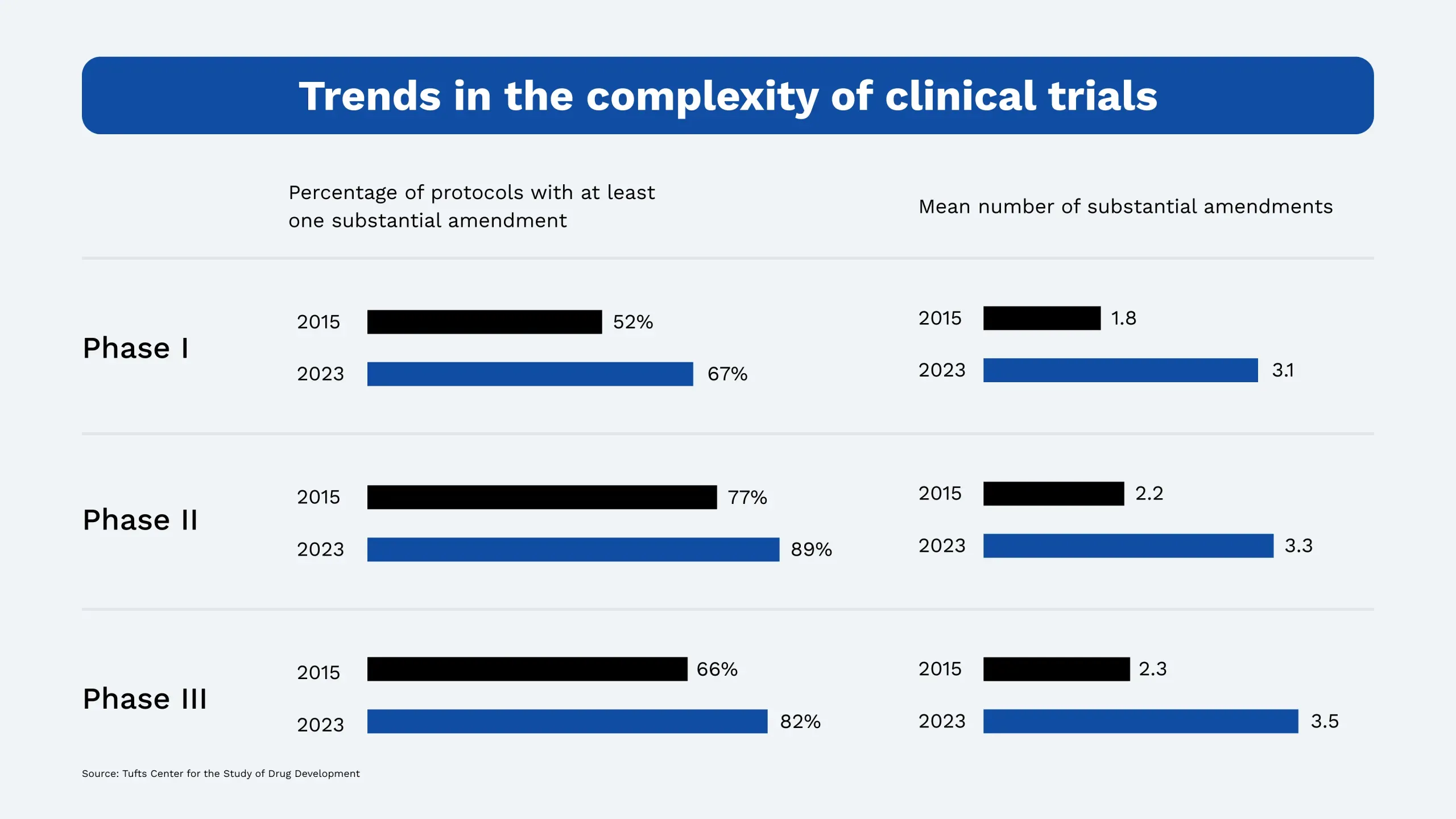
Innovation in drug development has accelerated dramatically in the past decade. Trials are more intricate, timelines are tighter, and protocols are evolving faster. According to recent data from the Tufts Center for the Study of Drug Development, 82% of Phase III protocols from 2018–2021 included at least one substantial amendment, up from 66% in 2013–2015. The average number of amendments per protocol jumped from 2.3 to 3.5, increasing complexity, cycle times, and demand on clinical teams.
These shifts require a fundamental change in how organizations plan, manage, and deploy their clinical workforce. Relying on siloed spreadsheets or static headcount models is no longer enough. Success now depends on intelligent, adaptive resource management — powered by real-time data, predictive analytics, and cross-functional coordination.
As organizations are asked to do more with fixed or shrinking resources, a clear understanding of capacity and strategic deployment has become essential.
Common issues in resource management
Despite best efforts, life sciences organizations continue to struggle against three forces: inefficient processes, inadequate infrastructure and tools, and limited ability to identify and address underlying reasons for such inefficiency.
Inefficient processes – Resourcing practices remain decentralized, with redundant tracking and inconsistent reporting across functions requiring significant manual effort
Inadequate tools & infrastructure – Many organizations rely on outdated or fragmented systems that offer limited analytics, lack integration, and fail to enable proactive decision-making
Limited visibility and value – Reports often fail to provide actionable insight into resource capacity, leaving managers unable to match demand with available talent. As a result, resourcing can drift out of alignment with operational goals
These challenges are often symptoms of a deeper capability gap. While most organizations recognize the importance of resource management, few have built the integrated infrastructure, processes, and governance needed to manage resources proactively and at scale. Addressing this gap requires a structured approach to capability development — one that evolves with organizational needs and technological change.
Developing resource management maturity
Creating a robust resource management capability is a journey. It starts with building foundational visibility into data, roles, and processes, and progresses into a dynamic system that supports real-time, strategy-aligned decision-making. Across the industry, Acquis’ Life Sciences has observed a consistent pattern of maturity that organizations follow as they evolve RM capabilities:
Common scenarios
Stage I | Decentralized resource management
At this stage, functional teams manage their own data collection, analysis, and reporting. While this may offer local flexibility, it leads to inconsistent assumptions, limited data quality checks, and minimal cross-functional visibility. Forecasting and capacity planning are often based on incomplete or outdated information, making it difficult for leaders to allocate resources effectively or identify future gaps.
Stage II | Centralized and optimized resource management
This is the ideal organizational model. Here, organizations mature their infrastructure, establish common processes, and develop reporting cadences that offer meaningful insights. Leadership gains visibility into bandwidth, future demand, and tradeoffs; feedback loops and predictive tools support real-time scenario planning; and centralized RM unlocks the ability to make data-driven decisions about where to invest resources and how to balance workload across teams.

Closing the gap: strategies for strengthening resource management
Organizations seeking to shift from a decentralized model must apply several foundational strategies that align systems, people, and processes:
Conduct a holistic diagnostic
First, understand how resource management is practiced across the organization. This includes mapping current tools and systems, reviewing reporting outputs, and collaborating stakeholders across functions. A thorough diagnostic will go beyond the data to uncover behavioral and cultural factors that affect processes and identify both structural inefficiencies and unmet user needs, creating a roadmap for targeted improvements.
Define the building blocks of work
Resource management is built on foundational “blocks” that shape how teams operate:
Roles: What are the core responsibilities of each role? Are teams focused on high-value activities?
Processes: Where do delays or redundancies occur? Are handoffs clear?
Systems: Do tools support visibility and forecasting, or do they add friction?
Decisions: Who makes resourcing calls, and what informs those choices?
Clarifying and aligning these elements allows organizations to remove friction, reduce redundancy, and redeploy effort toward high-impact work.
Develop tailored resourcing models
Generic planning models rarely reflect the complexity of real-world clinical operations. By tailoring models based on function-specific workflows, trial phase variation, and regional differences, organizations can forecast more accurately. Custom algorithms — informed by collaborative conversations, historic data, and workflow insights — can help predict demand, identify capacity constraints, and dynamically reallocate resources. Additionally, by developing fit-for-purpose dashboards, you enable functional leaders to monitor and adjust plans in real time.
Implement with change in mind
Even the best-designed RM system will fail without adoption. Implementation must include structured change management, with emphasis on communication, governance, and training. Teams need clear roles, repeatable processes, and digital fluency to interact with new tools. Leaders should prioritize early wins, pilot programs, and user feedback to build trust and sustain momentum.
Levers for long-term success
To future-proof RM capabilities, organizations should embrace continuous improvement, technology integration, and strategic alignment through:
Predictive analytics – Leverage AI and machine learning to simulate trial timelines, forecast enrollment, and identify bottlenecks. These tools allow teams to plan proactively and rebalance resources in real time, reducing cycle time and trial delays.
Feedback loops – Build structured mechanisms for feedback from end users and functional leaders. Regularly revisit forecast assumptions, dashboard utility, and system usability to ensure the RM capability continues to evolve with business needs.
Resourcing to value – Shift the focus from effort tracking to impact. Eliminate low-value work, standardize routine tasks, and redeploy resources to high-priority activities. Removing even 5-20% of nonessential tasks can free up capacity without increasing headcount.
Workforce development – Invest in training and upskilling to close the digital literacy gap. As trial execution becomes more decentralized and data-driven, the workforce must be equipped to use analytics platforms, collaborate virtually, and make informed decisions using real-time data.

Conclusions and considerations
As clinical trials evolve, so does the need for strategic resource management. Organizations that develop mature, proactive RM capabilities stand to gain significant value: faster study execution, greater visibility into resource capacity, and more confident decision-making.
The outcomes are clear. When resource management is approached with the right structure and mindset, life sciences organizations benefit from:
Improved coordination across functions
Enhanced visibility and forecasting
Proper team deployment to high-value priorities
Continued refinement of tools and processes
Reduced number of low-value tasks and increased capacity
Implementing this kind of capability transformation is no small feat — it requires expertise in analytics, systems thinking, and change management. If your organization is ready to build a more resilient, forward-looking RM function, Acquis can help.
Acquis partners with life sciences companies to design and implement resource management solutions tailored to their goals, structure, and operational maturity — helping teams unlock capacity, accelerate clinical delivery, and future-proof their operations.
Learn more about Acquis’ Life Sciences Advisory →
Originally published December 2020 | Refreshed April 2025